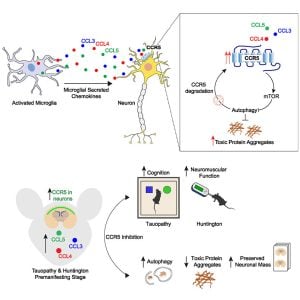
A common characteristic of neurodegenerative diseases such as Huntington’s disease and various forms of dementia is the build-up in the brain of clusters—known as aggregates—of misfolded proteins, such as huntingtin and tau. These aggregates lead to the degradation and eventual death of brain cells and the onset of symptoms.
One method that our bodies use to rid themselves of toxic materials is autophagy, or ‘self-eating,” a process whereby cells ‘eat’ the unwanted material, break it down and discard it. But this mechanism does not work properly in neurodegenerative diseases, meaning that the body is no longer able to get rid of the misfolded proteins.
In a study published today in Neuron, a team from the Cambridge Institute for Medical Research and the UK Dementia Research Institute at the University of Cambridge has identified a process that causes autophagy not to work properly in the brains of mouse models of Huntington’s disease and a form of dementia—and importantly, has identified a drug that helps restore this vital function.
The team carried out their research using mice that had been genetically-altered to develop forms of Huntington’s disease or a type of dementia characterized by the build-up of the tau protein.
Using mice, the team showed that in neurodegenerative diseases, microglia release a suite of molecules which in turn activate a switch on the surface of cells. When activated, this switch—called CCR5—impairs autophagy, and hence the ability of the brain to rid itself of the toxic proteins. These proteins then aggregate and begin to cause irreversible damage to the brain—and in fact, the toxic proteins also create a feedback loop, leading to increased activity of CCR5, enabling even faster build-up of the aggregates.
When the researchers used mice bred to ‘knock out’ the action of CCR5, they found that these mice were protected against the build-up of misfolded huntingtin and tau, leading to fewer of the toxic aggregates in the brain when compared to control mice.
This discovery has led to clues to how this build-up could in future be slowed or prevented in humans. The CCR5 switch is not just exploited by neurodegenerative diseases—it is also used by HIV as a ‘doorway’ into our cells.
The team used maraviroc to treat the Huntington’s disease mice, administering the drug for four weeks when the mice were two months old. When the researchers looked at the mice’s brains, they found a significant reduction in the number of huntingtin aggregates when compared to untreated mice.
The same effect was observed in the dementia mice. In these mice, not only did the drug reduce the amount of tau aggregates compared to untreated mice, but it also slowed down the loss of brain cells. The treated mice performed better than untreated mice at an object recognition test, suggesting that the drug slowed down memory loss.
Professor Rubinsztein added: “We’re very excited about these findings because we’ve not just found a new mechanism of how our microglia hasten neurodegeneration, we’ve also shown this can be interrupted, potentially even with an existing, safe treatment.”
“Maraviroc may not itself turn out to be the magic bullet, but it shows a possible way forward. During the development of this drug as a HIV treatment, there were a number of other candidates that failed along the way because they were not effective against HIV. We may find that one of these works effectively in humans to prevent neurodegenerative diseases.”